With countless cough medicines available, choosing the right one requires understanding its purpose and precautions. Neo Codion, a codeine-based solution, targets specific cough types. Learn from pharmacist Nguyen Hoang Bao Duy about its proper use, risks, and expert recommendations in this comprehensive guide.
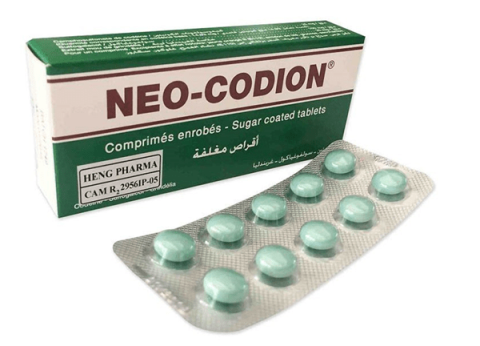
Active ingredients: Codeine camphosulphonate (25mg), sulfogaiacol (100mg), Grindelia soft extract (20mg).
Common brand variants: Neo-Cod F, Neo-codin.
Table of Contents
What is Neo-Codion?
Neo Codion, a respiratory tract medication, combines three active components to suppress persistent dry coughs. Manufactured by Bouchara Recordati (France), it's available in sugar-coated tablets.
Primary Uses
Neo Codion treats adult dry coughs unresponsive to other remedies. Note: Requires prescription.
| Component | Role |
|---|
| Codeine | Suppresses cough reflex |
| Sulfogaiacol | Reduces throat irritation |
| Grindelia extract | Natural expectorant |
When to Avoid Neo Codion
- Under 18 years old
- Pregnancy/breastfeeding
- Asthma-related cough
Cost Analysis
Average price: 95,000 VND/box (≈ $3.90 USD). Contains 20 tablets.
Dosage Guidelines
| Group | Dose | Frequency |
|---|
| Adults | 1 tablet | Every 6 hours (max 4/day) |
| Elderly | ½ tablet | Adjust based on tolerance |
Administration tip: Can be taken with/without food.
Reported Side Effects
- Common: Nausea, constipation (25% users)
- Rare: Urinary retention, tachycardia

Critical Safety Notes
- Avoid alcohol – increases drowsiness risk
- Monitor liver/kidney patients closely
- Discontinue if rash or breathing issues occur
Featured Snippet: Key Recommendations
- Never exceed 4 tablets daily
- Store below 30°C (86°F)
- Seek help if overdose symptoms appear
Final note: Always consult healthcare providers before use. For overdose concerns, contact Vietnam Poison Control Center (+84-24-3826-5115) immediately.