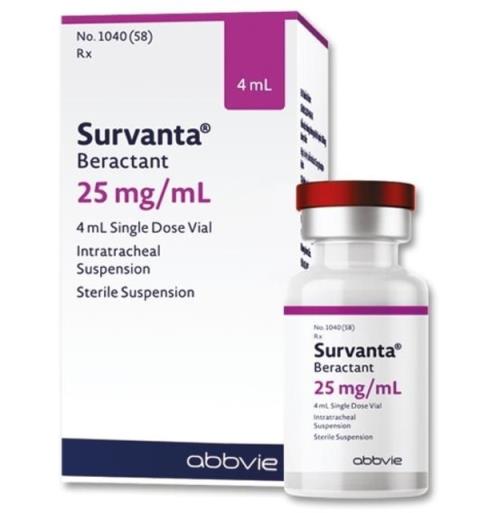
Survanta (beractant) is indicated for the prevention and treatment of respiratory distress syndrome in premature infants. So how is Survanta used and what should be noted when using it? Let's learn the necessary information about drugs through the following SignsSymptomsList article.
Active ingredient: beractant.
Drugs with similar ingredients: Curosurf, Alvofact ...
content
What is Survanta (beractant)?
Ingredient
Each vial contains 100mg of phospholipids. The specific chemical composition of beractant is as follows:
- Total phospholipids: about 25mg/mL.
- Free fatty acids: 1.4 – 3.5mg/mL.
- Triglycerides: 0.5 - 1.75mg/mL.
- Protein: 0.1 – 1.0mg/mL.
Description
The form of Survanta (beractant) suspension is an opaque liquid, white to light brown in color. Sterile, non-pyrogenic, suspension for endotracheal administration, extracted from bovine lungs.
Find out drug information Survanta (beractant)
Mechanism
Endogenous surfactants in the lung have the following effects:
- Reduces surface tension in alveolar sacs during respiration
- Stabilizes alveoli against collapsing due to pleural pressure.
Pulmonary surfactant deficiency causes respiratory distress syndrome (RDS) in neonates . Beractant in Survanta supplements pulmonary surfactant and maintains lung surfactant in these pediatric patients.
Indications for the drug Survanta (beractant)
Survanta (beractant) is indicated for the prevention and treatment of respiratory distress syndrome (RDS) (inner membrane disease also known as Hyalin membrane disease) in premature infants. Beractant significantly reduced RDS morbidity and mortality as well as air leak complications in these pediatric patients.
- Prevention: In premature infants weighing less than 1250g or with evidence of surfactant deficiency, administer beractant as soon as possible, preferably within 15 minutes of birth.
- Treatment: For the treatment of pediatric patients with RDS who have been confirmed radiographically and require the use of artificial ventilation, administer beractant as soon as possible, preferably within 8 hours of birth.
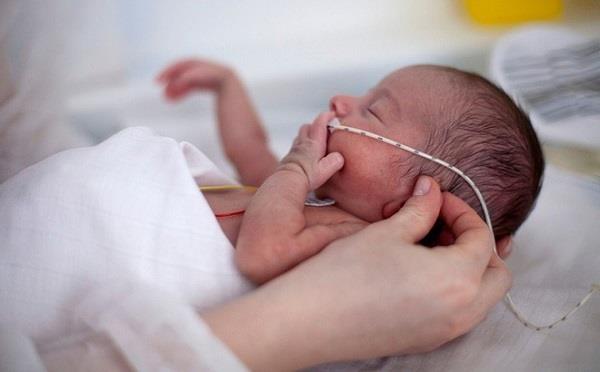
Survanta (beractant) is used in the treatment of respiratory failure in infants
Instructions for use Survanta (beractant)
Note:
- Survanta (beractant) is for endotracheal use only.
- Beractant should be used under the supervision of healthcare professionals experienced in intubation, ventilator management, and the care of premature infants.
- Oxidation may occur during treatment with beractant.
Dosage: Each dose of beractant is 100 mg of phospholipids/kg of birth weight (4 mL/kg). Up to 4 doses of beractant can be administered within 48 hours of birth. The interval between doses should not be less than every 6 hours.
How to use:
- Visually inspect the suspension for discoloration. Normal color is white thanks to light brown.
- If the suspension settles during storage, swirl the container gently (be careful not to shake). Foam may occur during transportation and is a natural feature of the product.
- Beractant must be kept refrigerated between 2°C and 8°C. Before use, the medicine should be warmed in the palm of your hand for at least 8 minutes or left at room temperature for at least 20 minutes. Absolutely do not use other methods of artificial warming. If used for preventive purposes, it should be prepared before the baby is born.
- For intratracheal administration through a drip catheter with a terminal size of 5F. Do not aspirate pediatric sputum within 1 hour of dosing, unless there are signs of significant airway obstruction.
Contraindications of Survanta (beractant)
There are no known contraindications to Survanta (beractant).
Precautions while using Survanta (beractant)
- Survanta (beractant) is for endotracheal use only.
- It should be used under the supervision of medical personnel experienced in intubation, ventilator control, and the care of premature infants .
- Regularly monitor oxygen & carbon dioxide levels.

The use of Survanta requires medical supervision and support
Survanta (beractant) side effects
Undesirable effects that Survanta (beractant) may cause for children such as transient bradycardia, decreased oxygen saturation, endotracheal tube reflux, cyanosis, vasoconstriction, increase/decrease in blood pressure, endotracheal tube obstruction, hyper/decreased blood carbon dioxide, asphyxiation.
Survanta (beractant) drug interactions
There have been no reports of Survanta (beractant) interactions with other drugs and other types of interactions.
Treatment of overdose Survanta (beractant)
There have been no reports of overdose with Survanta (beractant) . Based on animal studies, overdose can cause acute airway obstruction. Symptomatic treatment is necessary.
Rales and wet rales may occur transiently after the use of beractant and are not due to an overdose. Do not require endotracheal sputum suction or other support unless obvious signs of airway obstruction occur.

How to handle an overdose of Survanta?
For pregnant and lactating women
There are no clinical data on the use of Survanta (beractant) in pregnant and lactating women.
How to store Survanta (beractant)
- Store Survanta (beractant) at a temperature between 2 degrees Celsius and 8 degrees Celsius.
- Do not shake the medicine.
- Keep out of reach of children.
- Read the instructions carefully before use.
Survanta (beractant) is indicated for the prevention and treatment of respiratory distress syndrome in premature infants. Above is the reference information from SignsSymptomsList about Survanta drug. If you have any concerns, contact your doctor or pharmacist for specific advice.